| Name | Canagliflozin |
| Classes |
Antidiabetic Agent Metabolic Agent SGLT-2 Inhibitor |
| Diseases |
Type 2 Diabetes Mellitus Hormonal Disorder |
Canagliflozin
Canagliflozin is an antidiabetic drug from the class SGLT-2 inhibitors. Canagliflozin inhibits sodium-glucose like transporter 2, a transport protein that reabsorbs sodium & glucose from urine into the blood. As a result, glucose is excreted in urine and blood glucose level drops.
Canagliflozin is indicated in the management of type 2 diabetes mellitus.
The recommended starting dose is 100 mg once daily, taken before the first meal of the day. Dose can be increased to 300 mg once daily in patients tolerating Canagliflozin 100 mg once daily who have an eGFR of 60 mL/min/1.73 m2 or greater and require additional glycemic control. Assess renal function before initiating and periodically thereafter. Limit the dose of Canagliflozin to 100 mg once daily in patients who have an eGFR of 45 to less than 60 mL/min/1.73 m2. Initiation or use of Canagliflozin is not recommended if eGFR is below 45 mL/min/1.73 m2 .
Commonly associated side effects are-
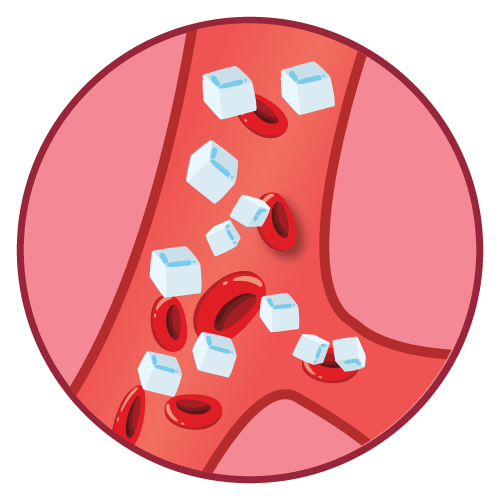
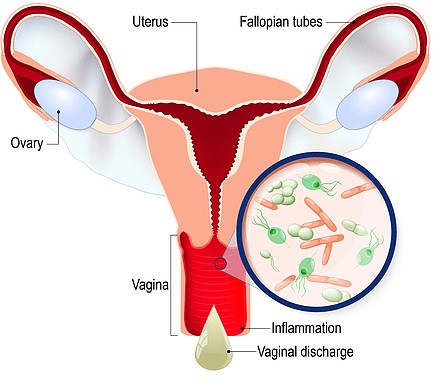
- Vaginal candidiasis
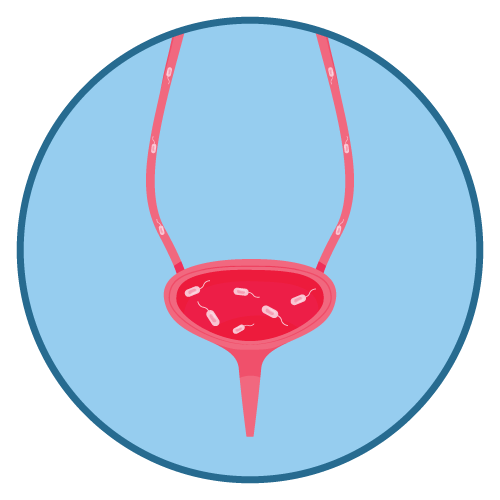
- Urinary tract infections
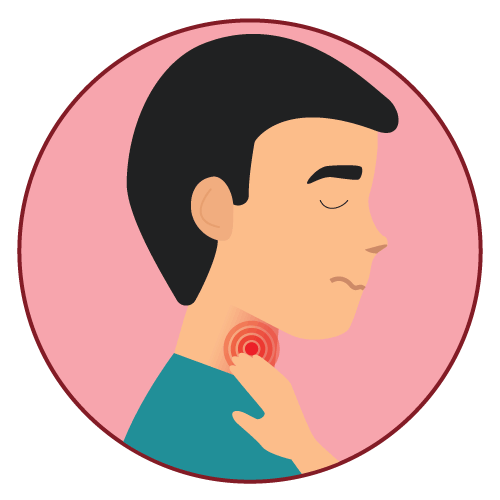
- pharyngitis
- In patients with type 2 diabetes who have established cardiovascular disease (CVD) or at risk for CVD, Canagliflozin has been associated with lower limb amputations, most frequently of the toe and midfoot; some also involved the leg.
- Before initiating, consider factors that may increase the risk of amputation. Monitor patients receiving Canagliflozin for infections or ulcers of the lower limbs, and discontinue if these occur.
- Before initiating Canagliflozin, assess volume status and correct hypovolemia in patients with renal impairment, the elderly, in patients with low systolic blood pressure, or if on diuretics, ACEi, or ARB. Monitor for signs and symptoms during therapy.
- Consider temporarily discontinuing in settings of reduced oral intake or fluid losses. If acute kidney injury occurs, discontinue and promptly treat. Monitor renal function during therapy.
- Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated.
- Assess patients who present with signs and symptoms of metabolic acidosis for ketoacidosis, regardless of blood glucose level.
Contraindication
Contraindicated in patients hypersensitive to any component of the medication or-
None known.
Contraindicated in-
- Severe renal impairment (eGFR less than 30 mL/min/1.73 m2)
- Patients on dialysis
- End stage renal disease
 Bangla
Bangla English
English