| Name | Sevelamer (As Carbonate) |
| Classes |
Antidote Phosphate Binder |
| Diseases |
Hyperphosphorous Toxicity |
Sevelamer (As Carbonate)
Sevelamer carbonate is a phosphate binder medication that works by binding to phosphate in the gut and preventing it from being absorbed into the bloodstream. It is an anion exchange resin and lowers serum phosphorus concentrations without changing serum calcium concentrations.
Sevelamer carbonate is indicated for the control of serum phosphorus in patients with chronic kidney disease (CKD) on dialysis. It is also used to reduce serum phosphorus in patients with CKD who are not on dialysis.
- Starting dose is one or two 800 mg or two to four 400 mg tablets three times per day with meals.
- Adjust by one tablet per meal in two week intervals as needed to obtain serum phosphorus target (3.5 to 5.5 mg/dL).
- Serious cases of dysphagia, bowel obstruction, and perforation have been associated with sevelamer use, some requiring hospitalization and surgery. Sevelamer carbonate should be taken with meals to avoid gastrointestinal side effects.
- Patients with a history of bowel obstruction or major gastrointestinal surgery should use sevelamer carbonate with caution.
- Use of sevelamer carbonate may decrease absorption of vitamins D, E, and K, and folic acid. Patients should be monitored for deficiencies in these vitamins.
- Sevelamer carbonate may interact with other medications, so patients should inform their healthcare providers of all medications they are taking.
- Patients should be advised to take sevelamer carbonate at least 2 hours before or after other medications, as it may interfere with their absorption.
- Sevelamer carbonate has not been studied in pregnant women, so it should be used with caution in this population.
- It is not known if sevelamer carbonate is excreted in human milk, so caution should be exercised when administering it to nursing mothers.
Contraindication
Sevelamer carbonate is contraindicated in patients with known hypersensitivity to sevelamer carbonate or any of its components.
None known.
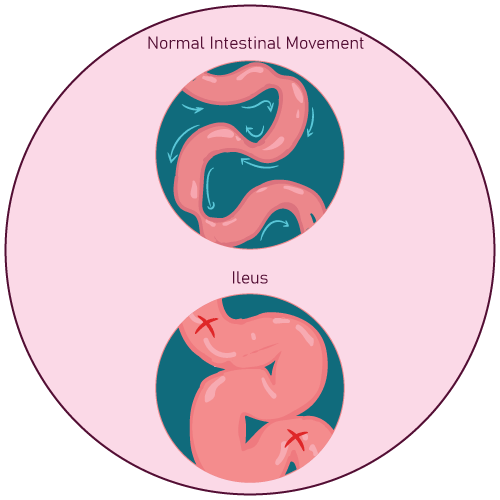
Sevelamer carbonate is contraindicated in patients with bowel obstruction & ileus.
 Bangla
Bangla English
English